


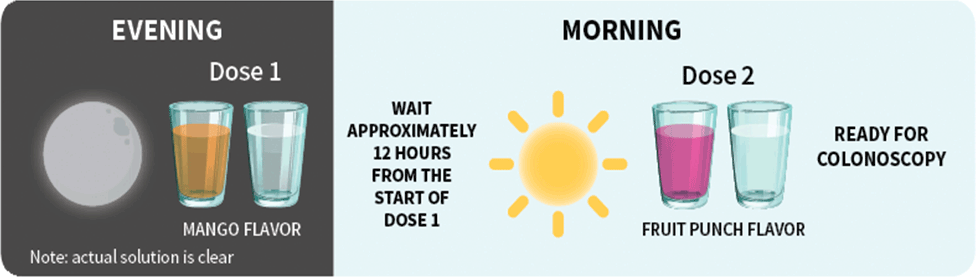
Patients should stop drinking liquids at least 2 hours before colonoscopy or as directed by their physician1

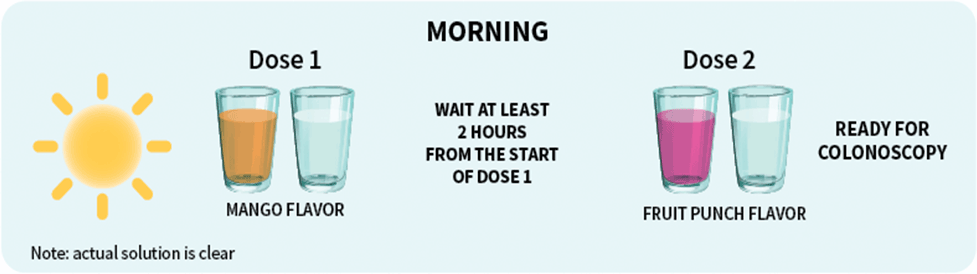
Patients should stop drinking liquids at least 2 hours before colonoscopy or as directed by their physician1
For each dose:
Tips for your patients
Please see the Instructions for Use.
*The “One-Day Morning Dosing Regimen” (as it is labeled in the Prescribing Information) is referred to as “Same-Day Morning-of-Colonoscopy Dosing”
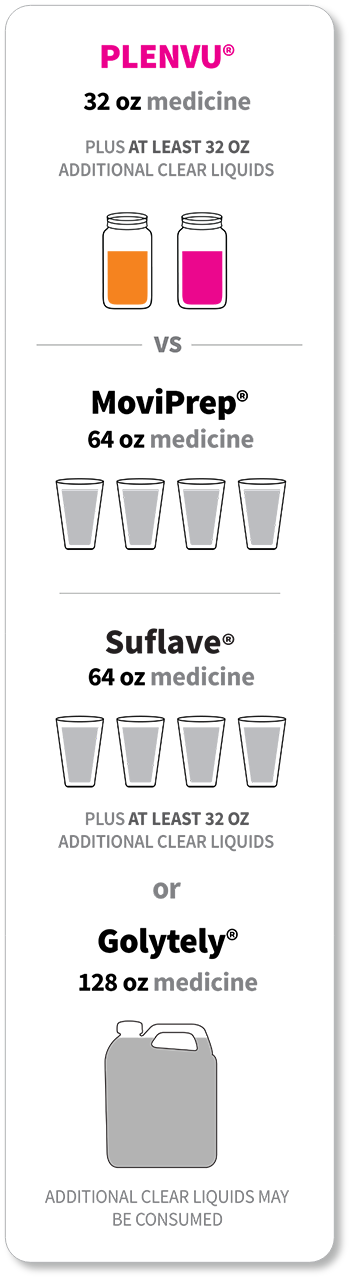
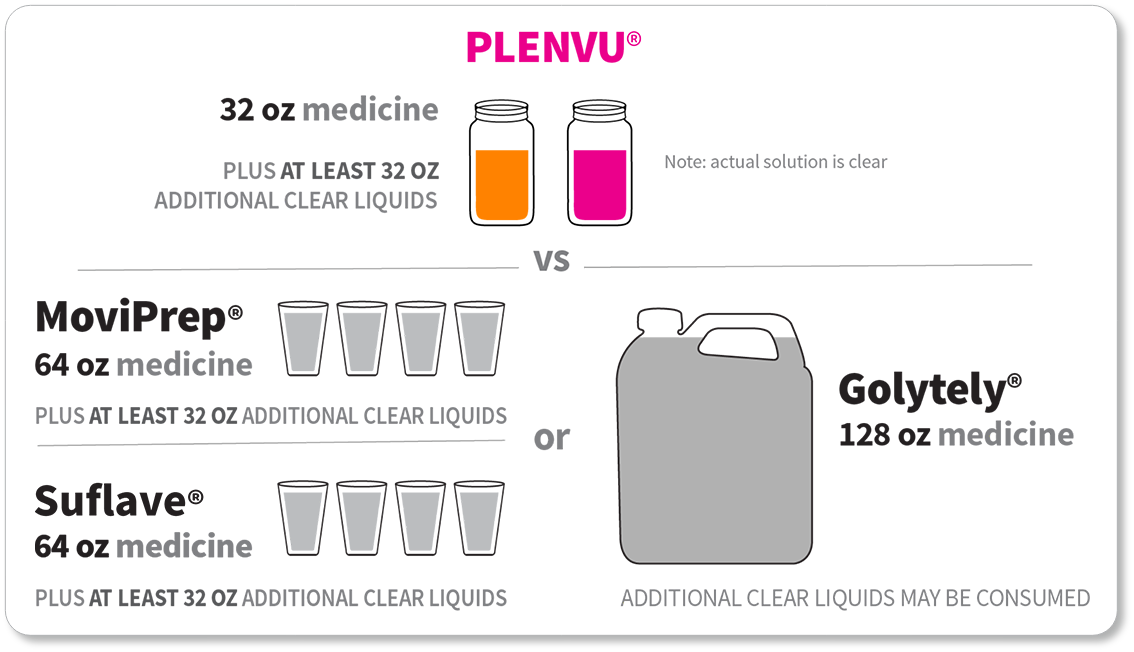
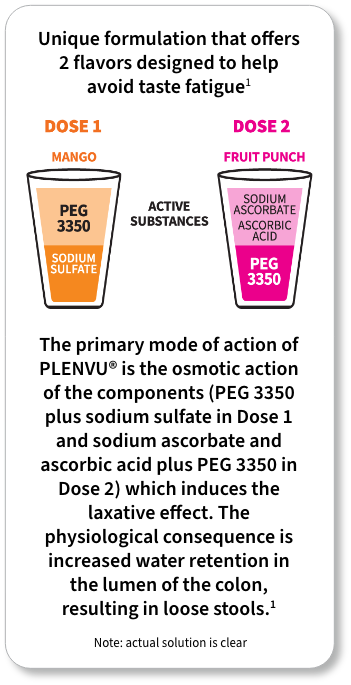
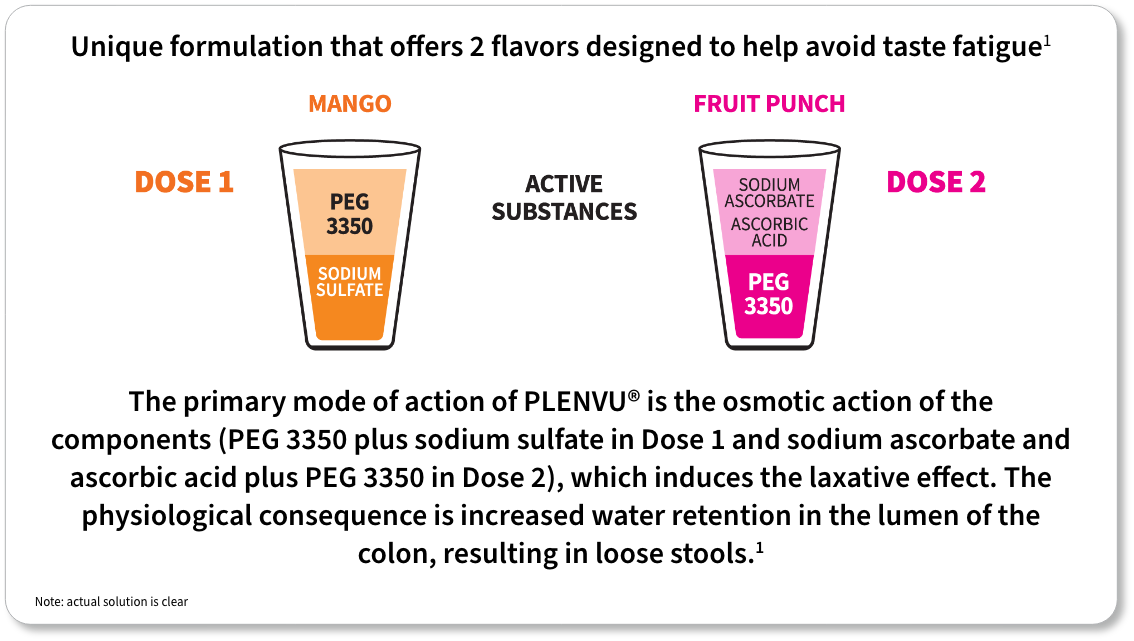
*The “One-Day Morning Dosing Regimen” (as it is labeled in the Prescribing Information) is referred to as “Same-Day Morning-of-Colonoscopy Dosing”
References: 1. Plenvu. Prescribing information. Salix Pharmaceuticals, a division of Bausch Health US, LLC; 2023. 2. MoviPrep. Prescribing information. Salix Pharmaceuticals; 2022. 3. Golytely. Prescribing information. Braintree Laboratories, Inc; 2021. 4. Suflave. Prescribing information. Braintree Laboratories, Inc; 2023. 5. Data on file. Braintree Laboratories, Inc. 6. Johnson DA et al. Gastroenterology. 2014;147(4):903-924. 7. Hassan C et al. Gastrointest Endosc. 2017;86(4):680-683.
PLENVU® (polyethylene glycol 3350, sodium ascorbate, sodium sulfate, ascorbic acid, sodium chloride and potassium chloride for oral solution) is an osmotic laxative indicated for cleansing of the colon in preparation for colonoscopy in adults.
To report SUSPECTED ADVERSE REACTIONS, contact Salix Pharmaceuticals at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please click here for full Prescribing Information.
PLENVU® (polyethylene glycol 3350, sodium ascorbate, sodium sulfate, ascorbic acid, sodium chloride and potassium chloride for oral solution) is an osmotic laxative indicated for cleansing of the colon in preparation for colonoscopy in adults.
PLENVU® is contraindicated in patients with gastrointestinal (GI) obstruction, bowel perforation, gastric retention, ileus, toxic megacolon, and hypersensitivity to any of its ingredients.